“Creating an Immune Tolerance Model Using an antiCD47 Single Domain Antibody” won 2nd place for the 2017 S. Klein Prize for Technical Writing.
Summary
Some diseases8, like haemophilia9, cystic fibrosis11, and growth hormone deficiency10, have treatments that consist of daily injections of a recombinant protein, which, by virtue of being foreign, can produce an immune response. When this happens, the treatment protein gets neutralized, rendering it ineffective. It has been shown previously that the immune response against a protein can be reduced by covalently attaching the protein to the surface of red blood cells extracted from a mouse and reinjecting them into the mouse. A4 is an antiCD47 single domain antibody that binds to the immune system protein CD477, which is heavily expressed in red blood cells. The overarching goal of this project is to show that conjugating A4 to another protein can cause a reduced immune response against both proteins, and that this immune tolerance system can be used to treat various diseases. Preliminary experiments in mice show that the immune response to an immunologically active single domain antibody (96G3m) is reduced when 96G3m is injected together with A4. To further assess the therapeutic potential of the immune tolerance system, we’ll use an immunologically active control protein (sortase) and utilize the mouse model to measure the response generated by the proposed A4-sortase construct in comparison to the response generated by just sortase. If the construct shows a reduction in immune response against sortase, we will use the system to show that we can extend the treatment potential of recombinant proteins in diseases like haemophilia9 and cystic fibrosis11. Such an accessible immune tolerance system would improve the prognosis of patients that suffer from these conditions without compromising cost of treatment13.
Specific Aims
Aim 1: To characterize the A4 immune tolerance system and assess its in-vivo efficacy using sortase as a proof-of-concept protein. We will generate the A4-sortase construct and validate its effectiveness in-vivo by injecting daily doses to a group of mice and comparing the immune response to a control group just injected with sortase. We will quantify the immune response through an ELISA carried out on the serum of the mice.
Aim 2: To test the A4 immune tolerance system and its ability to improve treatment using haemophilia as a proof-of-concept disease model. We will generate an A4-Factor VIII (FVIII) construct and validate its proper folding in vitro by showing it still binds to CD47 using Flow Cytometry. We will then inject daily doses of the construct to a haemophilia model mouse and measure the severity of the symptoms to ensure the activity of the construct as treatment. We will then also measure the strength of the immune response against FVIII over time, to see if the A4 immune tolerance system works and avoids inducing an immune response.
Background and Significance
Immune tolerance. Immune tolerance is the tactic of utilizing properties of the immune system to reduce or completely eliminate the immune response against a protein of interest1. The immune system defends an organism from infection by foreign pathogens through several different mechanisms2. The humoral immune response, one of these methods, is mediated by antibodies secreted by specialized B-cells known as plasma cells. The B-cell genetic repertoire, by virtue of its immense diversity, should contain sequences that produce antibodies that bind to self-proteins18. However, in order to avoid an immune response to the organism’s own cells, these B-cells get selected against and ultimately destroyed by the body19. This mechanism of immune selection against self is not perfectly understood, even though the mechanism is known to be shared with T-cells since they are the other major immune-response-mediating cell type2.
The key concept behind immune tolerance is tricking both B- and T-cells into thinking that a foreign protein is self, therefore selecting against B-cells and T-cells that would initiate an immune response against said protein3. By reducing the number of B-cells that recognize the protein of interest, fewer antibodies get produced that neutralize it, causing a measurable reduction in response. By establishing a mechanism in which we can suppress the immune response to certain foreign proteins, we can improve the treatment options to several disorders, like haemophilia, where the primary method of treatment is injection of a recombinant protein into the afflicted individual8.These proteins usually end up getting neutralized by the patient’s immune system,thereby rendering the treatment ineffective. However, by suppressing the immune response we can increase the efficacy of the treatments15.Immune tolerance has been shown to be accessible by covalently attaching proteins to red blood cells and reinjecting them into mice, with a measurable decrease in antibody titer against the protein of interest4. There are currently no standard treatments that use immune tolerance as treatment of some condition, and research into this area is developing possible treatments1,4 that, while effective, are expensive16 and invasive13. A practical and cheap method that uses immune tolerance is necessary if we ever want to use this as a therapy8. In this study, we propose using an intermediary protein that binds to red blood cells in order to replicate the effects of immune tolerance in a much more scalable fashion.
antiCD47-A4. CD47, which stands for cluster of differentiation 47, is a transmembrane protein which is involved in a broad swathe of pathways in every cell type in the body2. Of special note is the function of CD47 as an inhibitor of phagocytosis, preventing macrophages from attacking normal cells7. A4 is an anti-CD47 single-domain antibody that has been studied before for anti-tumor immunotherapy properties5. Since CD47 is expressed by every cell type, it exists on the surface of erythrocytes, which, as has been show, can be used to create an immune tolerance system as described in the previous section4. Preliminary studies confirm that daily injections of A4 produce a reduced immune response against it, and can also reduce response against other single-domain antibody fragments when injected in conjunction.
We want to show that by expressing A4 together with a protein of interest, we can achieve the same immune response reduction that has been observed before. Since the A4 immune tolerance system would be much more practical, it would offer an opportunity for grand-scale, more reliable treatments to diseases treated by protein replacement therapy, such as haemophilia A13. These more robust treatments would have a reduced chance of becoming neutralized by the immune system, leading to longer lasting effects and an improved prognosis8.
Preliminary Results
Treatment with A4 avoids an Immune Response. Previous studies have shown that proteins modified to bind to red blood cells are less immunogenic than their unmodified counterparts4. In these studies, the proteins were conjugated to a peptide recognized by a receptor in red blood cells. In order to examine if these results could be replicated using a single domain antibody fragment (VHH) that directly binds red blood cells, we studied the effects of injecting A4 in mice daily over a month. Figure 1a shows the average antibody response of mice injected with different VHHs quantified weekly over 4 weeks, while 1b presents the percentage of mice in each group with a high titer antibody response against VHHs over 4 weeks. Mice injected with A4 did not produce any detectable antibody response against it, while mice injected with an immunologically active VHH (96G3m) developed a high titer response. The data suggests that A4 avoids a humoral immune response, which usually occurs at most 14 days after exposure to the antigen.
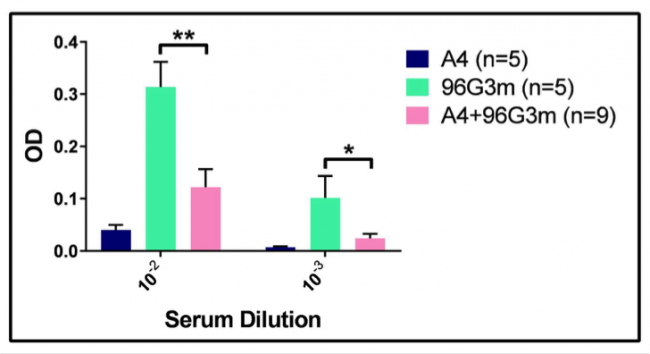
Treatment with A4+96G3m Reduces the Immune Response to 96G3m. Following the initial screen that showed that A4 does not produce a humoral immune response, we set on analyzing the consequences of this lack of response, and if it could be extended to similar proteins. Given that our irrelevant VHH 96G3m shares most of its aminoacid structure with A4, we evaluated if we could induce a reduction in the humoral immune response against it by a combined treatment with A4. To do so, we injected a group of mice daily for 4 weeks with both A4 and 96G3m at equal doses, as well as control groups with just A4 and 96G3m by themselves also at equal doses. Figure 2 shows the average antibody response of each group after 4 weeks of daily injections.
The data shows that the combined A4 and 96G3m treatment group developed an antibody response that is statistically significantly less than the response of the control 96G3m group. This data suggests that A4, through its mechanism of avoiding a humoral immune response, can suppress the development of a response to a different protein when injected together with it.
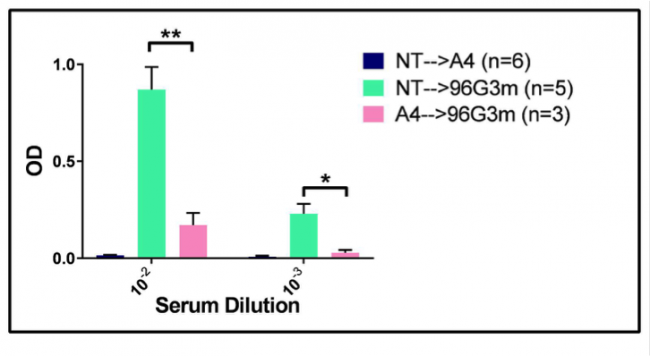
Treatment with A4 confers a reduced Immune Response to later treatment with 96G3m. After determining that A4 can confer reduced immunogenicity to 96G3m when injected together, we followed up on the exploration of A4 as an antibody response suppressor. In order to determine if the tolerance of antibodies against VHHs mediated by A4 occurs even after injections with A4 stop, we injected 96G3m daily into both mice that had received 4 weeks of daily A4 doses and mice that had received no treatment for 4 weeks. As a control, we also injected mice that had received no treatment for 4 weeks with daily A4 doses for 3 weeks. The data, shown in Figure 3, suggests that the immune tolerance conferred by A4 lasts even when the 96G3m challenge occurs after A4 dosing and not only when they go in parallel.
Research Design
Aim 1:To characterize the A4 immune tolerance system and assess its in-vivo efficacy using sortase as a proof-of-concept protein. Immune tolerance is the complete or partial reduction in immune response against an antigen1. By establishing how to suppress an immune response to certain foreign proteins, we can improve the treatment options to several disorders, like haemophilia16, where the primary method of treatment is injection of a recombinant protein into the afflicted individual15. Previous studies4 suggest that attaching proteins to erythrocytes effectively reduces the antibody response against the proteins, an effect that we seek to replicate by creating A4-protein constructs. Our model to prove this A4 immune tolerance system works will involve using sortase14 as our proof-of-concept protein, and we will characterize the system using this model. We are using sortase as our proof-of-concept protein given its small size of 18.7kDa, its foreignness to the mouse organism and consequent immunogenicity, and its well-established expression14.
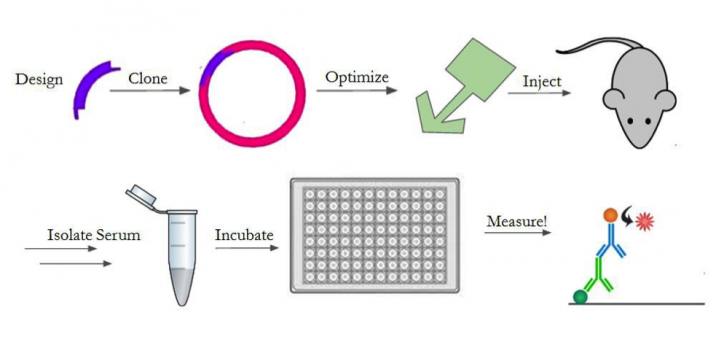
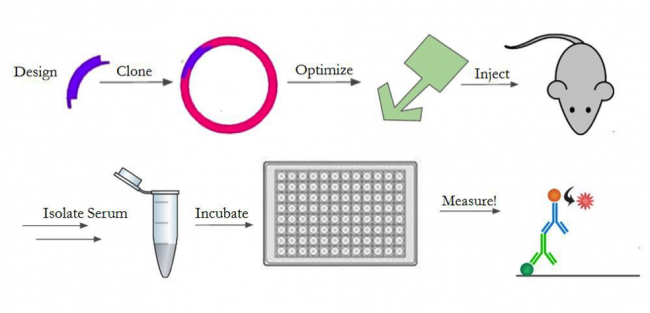
In order to test the response against our construct, we will design the gene, optimize its expression, purify and prepare it for injection, and inject it into mice. An outline of the entire process is shown in Figure 4. We will create the gene with a 6x histidine tag for downstream purification, as well as an LPETG motif for sortase reactions14,17. We chose a his-tag17 and sortase motif14 since the previous A4 characterization5 showed positive results from using these techniques to manipulate A4. For the in-vivo experiments, we determined we need 120mg of our protein construct. As such, we will generate the A4-sortase gene fragment and clone it into multiple expression vectors (pET30b, pET22b, pHEN6) to optimize its expression. These plasmids were chosen based on previous studies5,14 where A4 and sortase production were optimized successfully. We will then purify the construct using Nickel Affinity Chromatography17 and a size exclusion column. We will chemically conjugate it with a fluorescent dye (Alexa Fluor 647) to generate a probe. We will then take mouse spleen cells and use our fluorescent A4-sortase probe to show that the A4-sortase binds to CD47 in erythrocytes5 using fluorescence-activated cell sorting (FACS). After establishing that the construct still binds to red blood cells, we will inject 400ug, a typical immunogenic dose, of the construct daily into a group of 10 mice for 28 days in order to determine the strength of the immune response mounted against it.
We will then quantify the immune response against sortase in each of the mice. To do this, we will gather blood from the mice and isolate the serum every 7 days. We will test the serum for antibody presence by enzyme-linked immunosorbent assay (ELISA)4. After the 28 days, we will stop injecting the construct and change to injecting sortase daily, for 21 days, in order to assess if the change in immune response against sortase, if any, is permanent. We don’t expect for there to be any technical obstacles in achieving this goal, since we optimized the protocol when determining that A4 avoids inducing an immune response (Preliminary Results).
All these experiments will allow us to determine whether attaching foreign proteins to A4 will reduce or completely eliminate the immune response against the protein. The experiments will also give us an idea of the feasibility of an A4 immune tolerance system, given that we examine the process from the synthesis of the gene fragments to stitch together the sortase and A4, through the optimization of the protein expression, to the ease of purification before injection. So, through these experiments, we will completely characterize the A4 immune tolerance system.
One potential pitfall is all of the construct getting localized to tissues before making it to the bloodstream. One solution to this would be to alter the way of injecting the constructs, since intraperitoneal injections do not reach the blood immediately and this might hinder the efficacy of the treatment. If we change the method of injection to intravenous or retroorbital, we could potentially recover treatment usefulness. Another potential pitfall is the toxicity our constructs might have, since there is no precedent for an antiCD47-protein construct being injected into mice. To resolve this, we could reduce the amount of protein injected and the frequency of injections, since immune tolerance has been induced with doses of as little as 15ug4. This would be a significant reduction in dosage, which could eliminate any possible toxicity.
Aim 2: To test the A4 immune tolerance system and its ability to improve treatment using haemophilia as a proof-of-concept model. Having validated the immune tolerance system in Aim 1, we will look to determine the effectiveness of this model as a way to extend the treatment options for a range of diseases characterized by treatment consisting of injection of recombinant protein8. More specifically we will look at the immune responses mounted against recombinant Factor VIII protein in a haemophilia mouse model9. Haemophilia is a disease in which the endogenous Factor VIII is defective, and in the regular treatment option of injection of recombinant factor VIII, an antibody response against the recombinant protein neutralizes it and prevents it from being an effective treatment15. Using our system described in Aim1, we plan to reduce the antibody response against recombinant Factor VIII in a measurable way.
We will generate the A4-FVIII construct and clone it into several different protein expression vectors in order to maximize the protein expression and make enough of the construct in order to test it in vivo. This optimization step is necessary because each protein construct will have generally unpredictable expression rates in different vectors, so testing multiple of them is important to ensure we have the optimal vector for protein production. Like in Aim 1, we will then use a size-exclusion column and Nickel Affinity Chromatography17 in order to isolate a pure fraction of our protein. We will chemically conjugate our construct with Alexa Fluor 647 to synthesize a probe. We will then use mouse spleen cells and our probe to determine that our construct can still bind to CD47 by FACS. We do not expect any drawbacks in these experiments given that this protein expression and characterization has been well studied and optimized both before5 and in this study (Preliminary Results).
We will proceed to test our construct in vivo after purifying it, in the haemophilia A mouse model BALC/c X16-/16- in which the exon 16 of one of the FVIII subunits has been deleted and the protein is therefore not functional6. We will inject the construct daily for 28 days, and bleed the mice every 7 days in order to analyze specifically for treatment. We will isolate the serum from the blood then test it by ELISA against FVIII in order to measure the amount of antibodies against FVIII present in the system of the mouse4.
After the 28 days, we will inject the mouse with recombinant FVIII for 21 days and examine the serum afterwards in order to examine the change in antibody presence against FVIII, if any, after treatment with both the immunosuppressive construct and the immunogenic recombinant protein4. After this is done, we will measure the symptoms of the haemophilia disorder in the mice using the tail bleeding haemostatic assay12, in order to ensure that the A4-FVIII construct can restore clotting function as efficiently as recombinant FVIII by itself can. We will have a separate group of mice injected with recombinant FVIII as a control of both antibody response and clotting efficacy. The technical drawbacks of this part of the experiment focus on the assay performed in order to test the symptoms of haemophilia in the mouse model, since these mice are extremely fragile and, if treatment isn’t working properly, this testing will be fatal for the mice12. The mortality of the tail bleeding haemostatic assay is due to having to create in incision in the mouse tail and examine clotting where, in severely haemophiliac mice, the blood loss might be fatal due to lack of coagulation12. In order to address this drawback, we will just perform this after having performed our checkpoint bleeds to detect an immune response.
Altogether, these experiments would be a direct downstream application of our A4 immune tolerance system and would also serve as more evidence for using this A4-mediated immune tolerance as a standard part of treatment for similar diseases.

References
- Kontos, S., Grimm, A. J., & Hubbell, J. A. (2015). Engineering antigen-specific immunological tolerance. Current Opinion in Immunology, 35, 80-88.
- Owen, J. A., & Kuby, J. (2013). Kuby immunology. New York: W.H. Freeman.
- Griffith T. S., Ferguson T. A., Cell death in the maintenance and abrogation of tolerance: The five Ws of dying cells. Immunity 35, 456–466 (2011)
- Lorentz, K. M., Kontos, S., Diaceri, G., Henry, H., & Hubbell, J. A. (2015). Engineered binding to erythrocytes induces immunological tolerance to coli asparaginase. Science Advances, 1(6), e1500112.
- Sockolosky, J. T., Dougan, M., Ingram, J. R., Ho, C. C., Kauke, M. J., Almo, S. C., . . . Garcia, K. C. (2016). Durable antitumor responses to CD47 blockade require adaptive immune stimulation. Proceedings of the National Academy of Sciences Proc Natl Acad Sci USA, 113(19).
- Sabatino, D. E., Nichols, T. C., Merricks, E., Bellinger, D. A., Herzog, R. W., & Monahan, P. E. (2012). Animal Models of Hemophilia. Progress in Molecular Biology and Translational Science, 105, 151–209.
- Sosale, N. G., Spinler, K. R., Alvey, C., & Discher, D. E. (2015). Macrophage engulfment of a cell or nanoparticle is regulated by unavoidable opsonization, a species-specific ‘Marker of Self’ CD47, and target physical properties. Current Opinion in Immunology, 35, 107-112.
- Liras, A. (2008). Recombinant proteins in therapeutics: Haemophilia treatment as an example. International Archives of Medicine,1(1), 4.
- Ananyeva, N., Khrenov, A., Darr, F., Summers, R., Sarafanov, A., & Saenko, E. (2004). Treating haemophilia A with recombinant blood factors: A comparison. Expert Opinion on Pharmacotherapy, 5(5), 1061-1070.
- Johannsson, G., & Jørgensen, J. (2001). Safety aspects of growth hormone replacement in adults. Growth Hormone & IGF Research, 11(2), 59-71.
- Shah, P. L., Scott, S. F., Knight, R. A., Marriott, C., Ranasinha, C., & Hodson, M. E. (1996). In vivo effects of recombinant human DNase I on sputum in patients with cystic fibrosis. Thorax ,51(2), 119-125.
- Molina, E. S., Fujita, A., Sogayar, M. C., & Demasi, M. A. (2014). A quantitative and humane tail bleeding assay for efficacy evaluation of anti-haemophilic factors in haemophilia A mice. Haemophilia ,20(6).
- Auerswald, G., Prondzinski, M. D., Ehlken, B., Kreuz, W., Kurnik, K., Lenk, H., . . . Zimmermann, R. (2004). Treatment patterns and cost-of-illness of severe haemophilia in patients with inhibitors in Germany. Haemophilia,10(5), 499-508.
- Witte, M. D., Wu, T., Guimaraes, C. P., Theile, C. S., Blom, A. E., Ingram, J. R., . . . Ploegh, H. L. (2015). Site-specific protein modification using immobilized sortase in batch and continuous-flow systems. Nature Protocols,10(3), 508-516.
- Meeks, S. L., & Batsuli, G. (2016). Hemophilia and inhibitors: Current treatment options and potential new therapeutic approaches. Hematology,2016(1), 657-662.
- Klamroth, R. (2016). A new era of treatment for patients with haemophilia A? Hämostaseologie,37(1).
- Crowe, J., Döbeli, H., Gentz, R., Hochuli, E., Stüber, D., & Henco, K. (n.d.). 6xHis-Ni-NTA Chromatography as a Superior Technique in Recombinant Protein Expression/Purification. Protocols for Gene Analysis,371-388.
- Forsdyke, D. R. (2014). Lymphocyte repertoire selection and intracellular self/non-self-discrimination: Historical overview. Immunology and Cell Biology,93(3), 297-304.
- Übelhart, R., & Jumaa, H. (2015). Autoreactivity and the positive selection of B cells. European Journal of Immunology,45(11), 2971-2977.